Alexander G.Mrochek, ScDa, Alexander V.Frolov, ScDa, Tatiana G.Vaikhanskaya, PhDa, Alexander P.Voitovich, ScDb, Margarita V.Voitikova, PhDb, Olga P.Melnikova
aRepublican Scientific Practical Centre of Cardiology, Minsk, Belarus
bInstitute of Physics, Minsk, Belarus
Abstract
Background
The algorithm for precise differential diagnostics of atrial fibrillation and atrial flutter was recently developed.
Materials and methods
19 patients with atrial fibrillation and several digital ECGs from database.
Results
The algorithm developed is based on atrial ECG extraction by the blind source separation method and its spectral analysis. The number of spectral peaks in the 2-9 Hz range is calculated. Atrial flutter refers to one peak while atrial fibrillation refers to more than one peak.
Conclusions
This method was successfully tested in patients with atrial fibrillation or atrial flutter on several digital ECGs from database. The algorithm was 100% reliable.
Introduction
The electrical activity of the atria on the ECG shows a relatively low-voltage P-wave. Its location and measurement do not indicate a problem in the case of sinus rhythm. But during atrial fibrillation (AF) due to the chaotic electrical activity of the atria, the normal P-waves are replaced by a frequent, irregular series of pulses. Some of them are locked in the AV node, while others reach the ventricular myocardium, causing arrhythmic contractions, up to 200 per minute and more. Nonsynchronous atrial electrical activity leads to a worsening of its contractile function and a weakening of the cardiac output. AF is expressed in two different forms. They are atrial fibrillation (A-fbr) and atrial flutter (A-flt). A-fbr and A-flt are difficult to recognize on the ECG, in the tachysystolic phase. The real clinical problem lies in improving the accuracy AF is evident in about 33% of cases in persons hospitalized with heart rhythm disorders [1-3]. For the differential diagnosis of A-fbr/A-flt, an ECG with 12 standard leads is used. The signals in leads II, III and aVF are analyzed for the presence of P peaks. When the P peaks are absent and waves of different amplitudes and frequencies above 200 per minute are noted, A-fbr is diagnosed.
A-flt is diagnosed when these leads show more regular F-waves similar to "shark teeth" with a frequency of 240-320 per minute. This direct method reveals several disadvantages. First, the electrical activity power of the ventricles is a few times more powerful than the activity in the atria and it is disguised. Second, the low-amplitude atrial activity is masked by additional noise, which is noted in practically every ECG recording. Third, by the ECG records it is almost impossible to estimate the frequency and regularity of the atrial activity. With a repetition factor of conductivity 1:3 and 1:2 it becomes really difficult to distinguish A-flt from sinus rhythm and tachycardia. Taken together, these factors lead to a low reliability of the differential diagnosis of A-fbr/A-flt. Alternative diagnostic technologies based on the intracardiac electrophysiological investigations, ECG topography and tomography are expensive and not yet readily available for medical practice.
The aim of this study was to develop a method for the differential diagnostics of A-fbr/A-flt based on the spectral analysis of the atrial electrical activity and to conduct clinical trials.
Methods
In developing the method of differential diagnostics 19 ECGs (with 12 standard leads) from patients with AF, digitized at 1000 Hz, having a resolution of 22 bits, and 25 digital ECGs with paroxysmal AF, contained in an open database of ECG data of the Institute of Cardiotechnics, St. Petersburg, Russia (www.incart.ru), were studied. The duration of the ECG recordings was at least 1 minute. The ECG data previously cleared from 50 Hz interference, muscular and respiratory activity was then subjected to digital processing. Precision interpretation of the ECG data was performed using the Fourier-analysis and blind source separation algorithm, which are represented in the mathematical software MatLab 7.0. The study was performed on the 12-channel PC-based "Intecard electrocardiograph (Minsk, Belarus).
Atrial and ventricular electrical activity superimposed on one another in the time domain, produce a familiar ECG. To improve the diagnostic accuracy of the supraventricular arrhythmias it was desirable to separate the electrical activity of the atria. Previously used methods of digital filtering were not sufficiently accurate, as the frequency spectrum of the eliminated QRS-complex was varied, in the wide range of the arrhythmias noted. This was the main reason for the degenerating filtering quality. Some new approaches have recently been started for precision processing of the ECG. The principal component analysis method was successfully used to differentiate the fetal ECG from the mother’s ECG [4]. For atrial ECG identification the most commonly used method was the method of blind source separation (BSS) [5-7]. In this study, ECGs have been divided into their atrial and ventricular components by BSS method as well.
Generally, the problem is solved using the following method. A signal consisting of several independent sources S = [s1(t), s2(t)…sn(t)] is noted. There are n-signals X = [x1(t), x2(t)…x n(t)], each of which represents a linear mixture of sources S, related by the matrix A as X = AS . It is necessary to split the signals X, to make up U = [u1(t), u2(t)… un(t)], identical to S. To do this, a matrix W needs to be identified, which inverts the mixing process: U = WX. Estimation of the matrix is the essence of the blind source separation method. Isolation of the atrial components of the ECG is possible using the number of independent sources of reentry <9, as the number of independent sources are not to exceed the number of observed signals. A 12-lead ECG recording consists of 9 independent signals. Also, the sources of reentry are assumed to be independent of time and the time to pass to the surface electrodes is negligible. The algorithm BSS is adapted for ECG-signal processing.
Results
An extracted atrial ECG, additionally cleaned from noise and artifacts, was analyzed, and a variety of algorithms was built for differential diagnostics for supraventricular arrhythmias. Specifically, an algorithm was proposed for differential A-fbr/A-flt diagnostics. The scheme of this algorithm is seen in Fig.1.
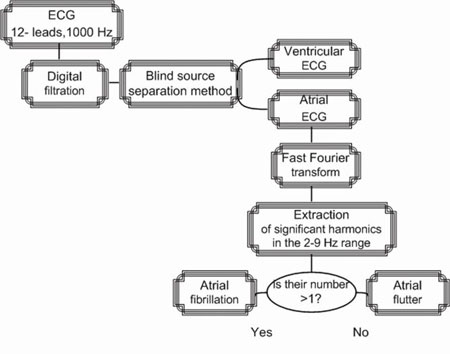
Fig.1. The algorithm of differential diagnostics of atrial fibrillation and atrial flutter by spectrum analysis of the atrial ECG
The selected atrial ECG was subjected to a frequency analysis by Fast Fourier Transform (FFT). Its range was analyzed from 2 to 9 Hz, as this was the range of wave activity with a great concentration of fibrillation. Then the number of harmonics (peaks) in this spectrum was counted. In the case of only one peak atrial flutter was diagnosed. Atrial fibrillation is diagnosed when the number of peaks observed is greater than one.
Clinical testing of the algorithm was conducted on digital ECG data recording the paroxysms of AF. Fig.2 shows an example of the differential diagnostics of a clinically verified AF episode in patient №149 from the ECG database of the Institute of Cardiotechnics, St. Petersburg, Russia. The left panel (a) shows the chest ECG leads V1-V6, below the corresponding atrial ECG Y1-Y6, the graph (b) represents the most powerful atrial lead Y1. A fragment of the atrial signal Y1 was processed by FFT using the Welch algorithm for 8012 points to the 4096-point Hamming window, with 50% overlap. The 2-9 Hz window of the spectrum was analyzed. This limitation improves the reliability, as this diapason contains only the atrial electrical activity. Spectral components outside this zone may reflect the heart rate and its high harmonics. The other components are not related to the atria. As evident from the lower right graph (c) in Fig.2 the spectrum of the atrial signal Y1 contains four harmonics. The maximum harmonic is seen at a frequency of 3.5 Hz. It is concentrated 34.1% of the spectrum energy. Therefore, it can be argued that the signal of atrial activity represents a mixture of all four independent sources of the reentry. Sinus activity is not represented here, as the ventricular activity is completely filtered out. Therefore, in this patient a few ectopic places of stimulation reentry were detected, by which any fibrillation waves of varying amplitudes and frequency could be seen. In this case, the exact clinical diagnosis is A-fbr.
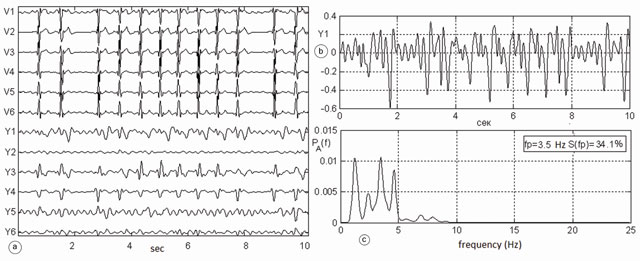
Fig.2. Episode of atrial fibrillation in a patient №149 from the database of the Institute of Cardiotechnics, St. Petersburg, Russia: a) precordial ECG leads V1-V6, at the lower end of this window extracted atrial ECG Y1-Y6; b) the most powerful atrial electrocardiogram in lead Y1 (scale enlarged), c) spectrum of atrial ECG, which contains four peaks in the range of 2-9 Hz
Fig.3 shows a fragment of a clinically verified episode of atrial flutter in the same patient №149. On the left panel (a) V1-V6 leads and the corresponding atrial signals Y1-Y6 are placed. Signal Y1 at the top right of the graph (b) is subjected to frequency analysis. Its spectrum is shown in the lower graph (c). The spectrum of atrial ECG shows only one peak at frequency =4.6 Hz. Its spectral power concentration is 87.3% of the total energy of the spectrum. Here, the excitation wave in the atria travels along a large circle of reentry that corresponds to the modern definition of atrial flutter. Atrial flutter occurs with a frequency of 276 beats per minute. Atrial flutter is diagnosed with 100% probability in this episode. Additionally, these examples show how atrial fibrillation is transformed into atrial flutter, and vice versa.
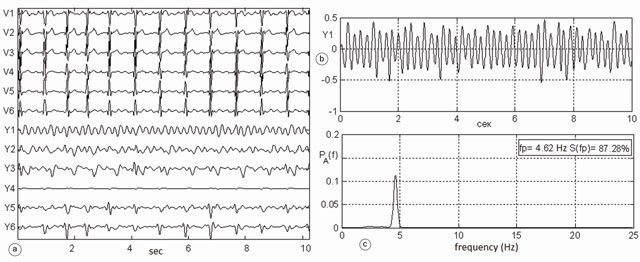
Fig.3. Episode of atrial flutter in the same patient #149 (the same database) a) precordial ECG leads V1-V6, at the lower end of this window atrial ECG Y1-Y6 b) the most powerful atrial electrocardiogram in lead Y1 (scale enlarged) c) spectrum of atrial ECG, containing a single peak at a frequency of 4.6 Hz; its spectral power concentration is 87.3% of the total spectrum power
Discussion
Besides the differential diagnostics of A-fbr/A-flt maximal frequency data it is possible to estimate the probability of spontaneous termination of atrial fibrillation or flutter. This has been proved by D. Hasser et al., [5]. To do this, the frequency in Hz must be transformed into the number of fibrillations per minute, in other words, multiply it by 60. It has been established that the higher the frequency the lower the probability of spontaneous termination of atrial fibrillation. Conversely, the lower the frequency of fibrillation the higher the probability of termination. The frequency analysis of the atrial ECG provided useful information on the number of independent sources of reentry. Also, information on the frequency and speed of conduction of excitation are roughly to estimate the size of the ectopic locus. However, this assumption calls for further study and is debatable.
Conclusions
The method developed in this study was based on blind source separation which offers a high reliability to extract atrial ECG based on the surface 12-lead ECG. Therefore, based on the spectral or wavelet analysis of an atrial ECG, one can build a variety of diagnostic algorithms of supraventricular arrhythmias. The frequency of ectopic activity can be estimated based on this spectrum.
An algorithm for differential diagnosis of atrial fibrillation and atrial flutter was successfully tested in 19 patients with atrial fibrillation, using several ECGs from the database at www.incart.ru. The algorithm is easy to use in choosing the alternative between the pharmacological and surgical correction of patients with atrial fibrillation. The technology is based on standard electrocardiographic equipment, currently widely available for clinical use.
References
1. Nedostup A, Blagova O, Bogdanova E, Platonova A. Noninvasive analysis of atrial and ventricular rhythm in atrial fibrillation: past, present and future of the method in clinical practice. Moscow: Media Sphere, 2005.
2. Syrkin A, Dobrovolsky A. Clinical management of patients with permanent atrial fibrillation: the current state of the problem. Moscow: Consilium Medicum, 2001.
3. ACC/AHA/ESC Guidelines for the management of patients with atrial fibrillation – executive summary. Europ. Heart Journal. 2006; 27 (16): 1979-2030.
4. Shulgin V, Tokarev A. The method of recording and analysis of fetal electrocardiogram during pregnancy. Radioelektronni i kompyuterni sistemi. 2008; 3 (30): 66-75.
5. Husser D, Stridh M, Sornmo L, Platonov P, Olsson S, Bolman A. Frequency analysis of atrial fibrillation from the surface electrocardiogram. Indian Pacing and Electrophysiology Journal. 2004; 4 (3):122-136.
6. Zarzoco V, Phlypo R, Meste O, Comon P. Signal extraction in multisensor biomedical recordings. Advances in biomedical engineering. 2008; 3:95-143.
7. Sung-Nien Y., Kuan-To C. Selection of significant independent components for ECG beat classification . Expert Systems with Applications: An International Journal. 2009; 36(2):2088-96.




